Procedure for registration of food supplements in accordance with the Decision on the Control on Production, Exportation-Importation of Food No. 856/MoH, dated 12 May 2006
This process is for those who want to import "food supplements". In this case you will need a registration certificate before proceeding with the normal import procedure.
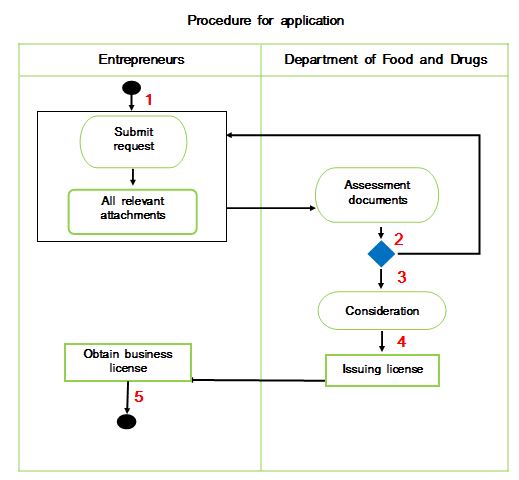
Procedure 1: Entrepreneurs need to fill out the application form according to the form of the Department of Food and Drug where can purchase the form at the Department of Food and Drug as details below:
Application form;
A request letters from entrepreneurs;
An authority letter from the Head of Office to assign a representative in Laos;
Sales license from the Food and Drug Administration of the exporting country;
Research certificate from the government or from a private laboratory certified to international standards, not older than 6 months from the exporting country;
Certificate from the Food and Drug Administration of the exporting country;
Product component certificate;
Label the product in Lao or print the Lao stickers on the box;
Good production certificate;
Registration of enterprises that can import and export food supplements;
Sample of original packaging (01 sample / item in original packaging).
Click for the checking list of application form.
Procedure 2: After completing the documents, the entrepreneur must submit the documents to the Department of Food and Drug, Ministry of Health. The Officers will check the documents before record the receipt number of documents, if not complete, the application will be return and clarified and instruct to complete the documents.
Procedure 3: After the documents are complete, they will be assessed and reviewed process by the Department of Food and Drug such as checking the accuracy of the documents, inspecting the place, food production method and collecting samples to send for research to verify the quality within the period of 4 months to release the license.
Procedure 4: The Department of Food and Drug will issue a registration certificate after careful consideration of the due process. Department of Food and Drug, Ministry of Health issues a sample Certificate, please Click
Procedure 5: Entrepreneurs can obtain a food supplement registration certificate from the Department of Food and Drug, Ministry of Health.
For more information, please contact:
Department of Food and Drug:
Thatkhao Village, Simuang Road, Vientiane Capital, Lao PDR
Phone: + (856-21) 214013
Fax: + (856-21) 214015
Website: www.fdd.gov.la
Email: [email protected]
Food and Drug Administration:
Simuang Village, Setthathilat Road, Vientiane Capital.
Tel: +856 21 255461
Fax: +856 21 254610
Website: www.bfdi.gov.la
| # | Title | Description | Issued By | File |
|---|---|---|---|---|
| 1 | Application for Registration of Food | Application for Registration of Food | Ministry of Health | |
| 2 | Certificate of Registration for Food Imports | Certificate of Registration for Food Imports | Ministry of Health |
| # | Name | Description | Measure Type | Agency | Comments | Legal Document | Validity To | Measure Class |
|---|---|---|---|---|---|---|---|---|
| 1 | Requirement to register for the risk food | For food only falls under the risk, it is necessary to register and present import plan to obtain a Certificate of Registration from the Department of Food of the Ministry of Health. For the list of risk food have not been drafted yet. | Registration Requirement | Ministry of Health | to control, monitor, inspect risk food, in order to ensure the safety of consumers and to avoid falsification by others. | Decision on the Control on Production, Exportation-Importation of Food, No. 856/MoH, date 12 May 2006 | 9999-12-31 | Good |
| 2 | Importer registering Requirement - Cosmetics | Cosmetic importers shall apply for the official registration to public health sector. | Registration Requirement | Ministry of Health | To guarantee the quality and safety of consumers and to avoid illegal importation of such products | Provision on the Quality Control of Cosmetic Products No. 2580/MOH | 9999-12-31 | Good |
| # | Process name | Process short name | Activity |
|---|
Please share your feedback below and help us improve our content.