China’s Ministry of Commerce, the General Department of Customs has issued relevant Notifications in order to stop and prevent the epidemic COVID -19. The notifications aim to introduce Medical Devices and Supplies Companies with Certification/Authorization in China. Therefore, there is the notification No. 5 in organizing and arranging medical equipment into systematic and the announcement, No. 12 in increasing and monitoring the quality of medical equipment.
The list of companies is as follows:
Table 1: Name list of Medical Devices and Supplies Companies with Certification/Authorization from other Countries.
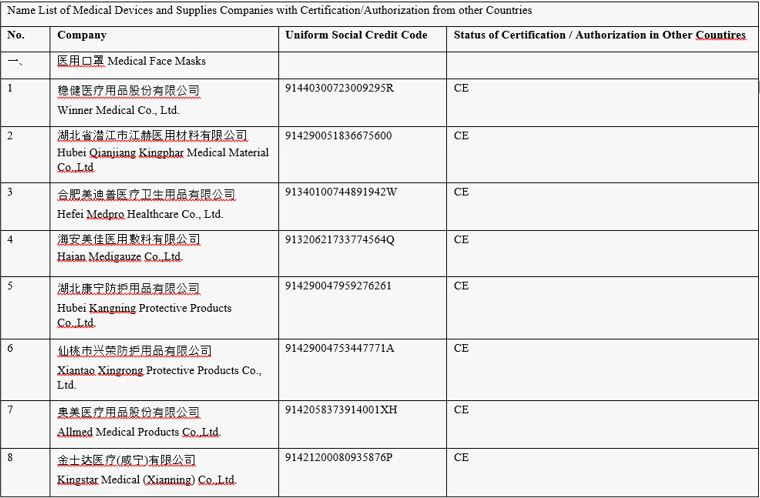
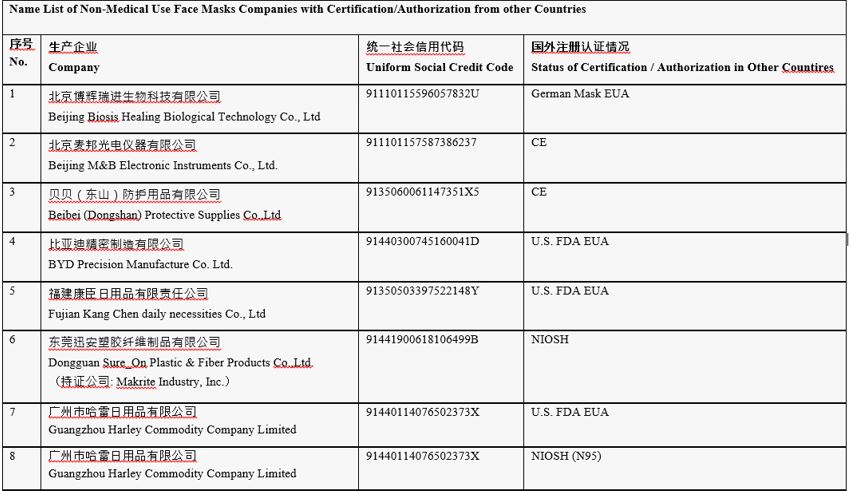
Table 2: Name List of Non-Medical use face masks companies with Certification/Authorization from other countries
Relevant resources:
- The Notification of the Qualified Chinese Pharmacy List, No. 094, dated 5 June 2020
- http://en.cccmhpie.org.cn/
- Name list of Medical Devices and Supplies Companies with Certification/Authorization from other Countries: http://en.cccmhpie.org.cn/Web/Content.aspx?queryStr=w7x08q7x15x15o3w8w1vS9z8w7x1X10x16x0X10x16o3w8w1u9v1u9vV6u9
- Name List of Non-Medical use face masks companies with Certification/Authorization from other Countries: http://en.cccmhpie.org.cn/Web/Content.aspx?queryStr=w7x08q7x15x15o3w8w1vS9z8w7x1X10x16x0X10x16o3w8w1u9v1u9vV5v5
Please share your feedback below and help us improve our content.
