The Law on Drug and Medical Products (Amended Version) No. 07/NA, dated 21st December 2011, and the Regulation on Governing Drug Registration No. 1441/MOH specify general principles for the import of raw materials, containers, drugs, and medical equipment.
Specific regulation that governs the import of raw materials, containers, drug, and medical equipment for production and distribution is the Notification on the Documents to Request for Import License of Raw Materials, Containers, Drug, and Medical Equipment for Production and Distribution No. 2660/16.FDD, dated 16 June 2016.
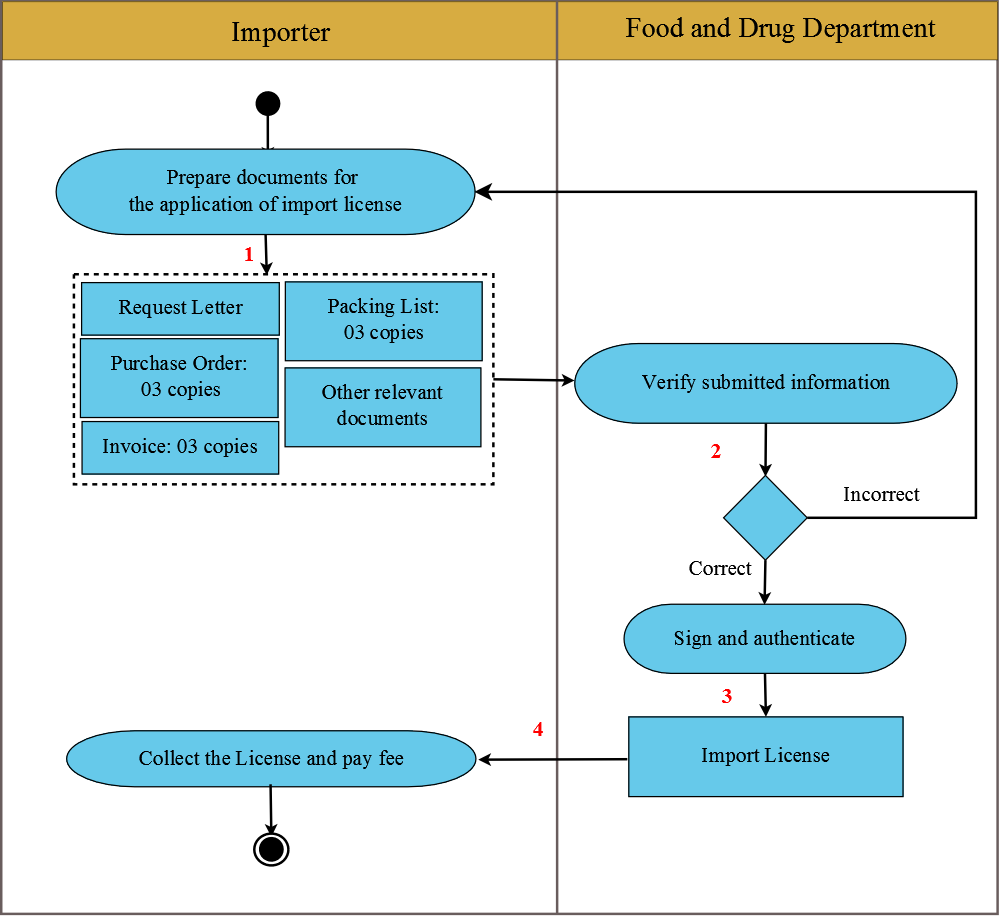
After an importer has successfully registered the drug and medical products import factory/company with Food and Drug Department, Ministry of Health, the importer can proceed on the procedure to import raw material, containers and drug (except for the import of medical equipment are not require to have factory/company registration) for production and distribution as follows:
1. Importer prepares documents for the application of import license, which consist of:
- Request Letter (download here)
- 03 copies of Purchase Orders
- 03 copies of Invoice (the Invoice shall indicate product lot number, manufacturing date, and expiry date. The name of products shall be clear and base on registered name, not only putting abbreviation or only product code)
- 03 copies of Packing Lists
- Besides the mentioned documents above, the import of each type of products requires different technical certificates as below:
- For the import of raw materials:
- Good Manufacturing Practice Certificate issued by product authority of manufacturing country or WHO Prequalification;
- Certificate of Analysis
- For the import of containers:
The containers to be imported shall be the containers that will support registered products. If the products are not being registered, they shall be approved as a sample of medicine production.
- For the import of Vaccines:
- Certificate of Lot Released issued by Drug Authority of manufacturing country
- GMP Certificate from manufacturing country and/or WHO Prequalification Certificate
- For the import of Medical Equipment
- Certificate of ISO 13485 from authority or governmental institution of manufacturing country
- Quality Analysis Certificate (for Consumable Devices) in compliance with WHO standards
- Used medical equipment are not allowed to import
Importer submits the mentioned documents above to Food and Drug Department, Ministry of Health.
2. Food and Drug Department considers the application in 03 weekdays.
- If the application is correct, the department will sign and authenticate the import license.
- If the application is incorrect, the department will notify the importer to re-submit the application.
3. Once the documents are complete and correct, Food and Drug Department will issue the License for the Import of Raw Materials, Containers, Drugs, and Medical Equipment for Production and Distribution.
4. Importer collects the License and pays the fee for 20,000 Kip at Drug Control Division, Food and Drug Department, Ministry of Health.
When obtaining the Import License, the importer can declare them with Food and Drug Authority at the border checkpoints and pay the fee for 100,000 Kip. After that, the importer can proceed to declare with customs authority to import raw materials, containers, drugs, medical equipment into Lao PDR.
Please share your feedback below and help us improve our content.
