Procedure for medication registration and application
Before importing medicines to treat diseases in Lao PDR, importers must first obtain a certificate from the Ministry of Health as states in the Decision on the Management of Narcotic Drugs, Nerve Affecting Substances and Basic Chemical Substances no. 456/MOF, dated 19 April 2006.
Procedure 1
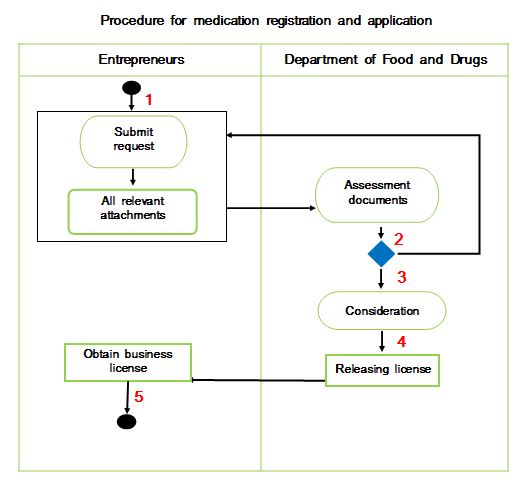
Entrepreneurs can purchase a certificate with the Department of Food and Drug, Ministry of Health. Click for reviewing application forms. Click for checking relevant documents which need to be submitted. Click for requiring documents which need to extend the drug registration.
Procedure 2
The submission of an import plan must be accompanied by some supporting documents. Document details may vary by product type. For further clarification, you can consult with the Department of Food and Drug, Ministry of Health.
Procedure 3
The Ministry of Health will request importers in order to provide a sample of the product for testing. The import sample application can download here for your reference.
Procedure 4
After the import plan is approved by the Ministry of Health, a registration certificate will be issued along with a number of conditions that the importer must comply with each time the importation is made. The certificate of registration is valid for one term. For each import, the importer must apply for an import license from the Ministry of Health.
Procedure 5
Applicant for registration or license. For registrations valid for two years, after two years the register must apply for extended license.
For more information: Department of Food and Drug, Ministry of Health
Thatkhao Village, Simuang Road, Vientiane Capital, Lao PDR
Phone: + (856-21) 214013
Fax: + (856-21) 214015
Website: www.fdd.gov.la
Email: info@fdd.gov.la
Please share your feedback below and help us improve our content.